Significant biosimilar activities this week include 01 May 2020 | AbbVie released its Q1 2020 financial results, reporting a 13.7% increase in US sales of Humira® (adalimumab)...


Significant biosimilar activities this week include 01 May 2020 | AbbVie released its Q1 2020 financial results, reporting a 13.7% increase in US sales of Humira® (adalimumab)...

Significant biosimilar activities this week include 23 April 2020 | Henlius Biotech announced it has received two EU GMP certificates related to its trastuzumab biosimilar HLX02....

Significant biosimilar activities this week include 21 Apr 20 | The Centre for Biosimilars reported that Xbrane Pharma was continuing clinical trials of its ranibizumab candidate...

Significant biosimilar activities this week include 14 April 20 | Mylan and Biocon launched Fulphila® (pegfilgrastim) in Australia. Fulphila® is indicated for the treatment of...

Significant biosimilar activities this week include March 20 | BiosanaPharma released the results of Ph I trials of BP001 (omalizumab), reporting comparability to Xolair®. 06...

Significant biosimilar activities this week include 30 Mar 20 | Biocad announced it had received registration certificates from Bosnia and Herzegovina for Acellbia® (rituximab)...

Significant biosimilar activities this week include March 20 | The UK High Court ruled filing errors in a Supplementary Protection Certificate (SPC) for Lucentis®...

Significant biosimilar activities this week include 16 Mar 20 | Teva and Celltrion launched Herzuma (trastuzumab) in the US. Herzuma is indicated for the treatment of breast...

Significant biosimilar activities this week include 09 Mar 20 | The FDA and FTC held a public workshop to further their efforts in creating a better biosimilar landscape in the...

Significant biosimilar activities this week include 02 Mar 20 | Celltrion launched Remsima SC, its subcutaneous infliximab product, in the UK. To support the launch,...

Significant biosimilar activities this week include 24 Feb 20 | The FDA launched a searchable version of the 'Purple Book', containing information about FDA licensed biological...

As we previously foreshadowed, the innovation patent system has been given its end dates: 25 August 2021: last date to lodge innovation patents; and 25 August 2029: date on which...

Significant biosimilar activities this week include 12 Feb 20 | Wockhardt announced divestment of assets to Dr Reddy's, under which 62 products and a manufacturing facility will...

Significant biosimilar activities this fortnight include 05 Feb 20 | Merck announced a new spin-off company which will incorporate products from its Women's Health, Legacy Brands...

Significant biosimilar activities this week include Jan 20 | Genentech and Roche commenced recruitment for Ph III clinical trials of their ranibizumab Port Delivery System (PDS)...
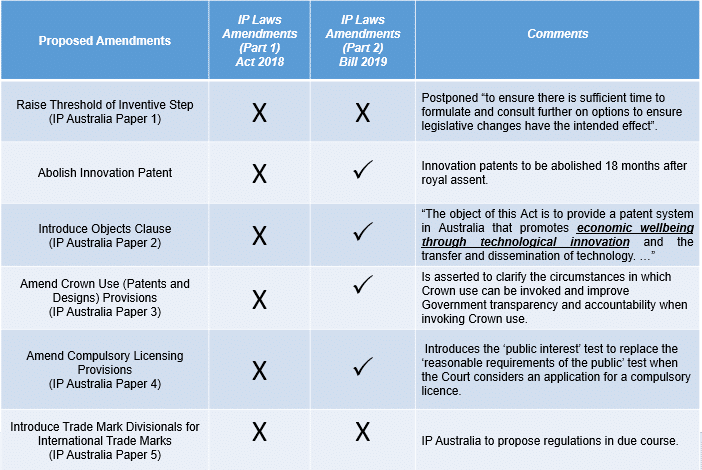
The Intellectual Property Laws Amendment (Productivity Commission Response Part 2 and Other Measures) Bill 2019 clears the Senate and heads to the House of Reps What is in the...

The Intellectual Property Office of New Zealand (IPONZ) has announced the introduction of excess claims fee, an increase in the examination fee, and increases in...
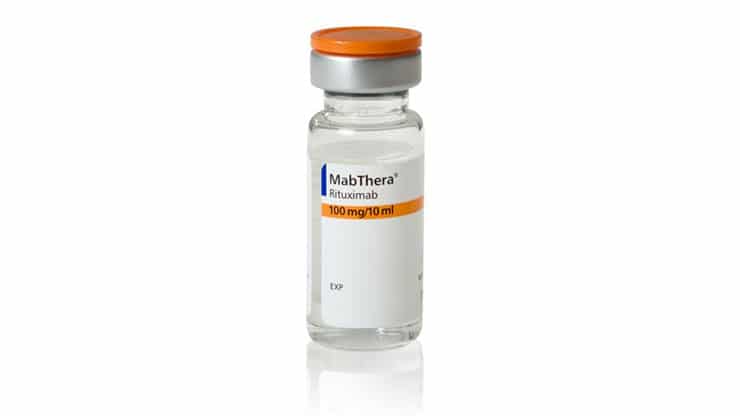
Today the Federal Court ordered an interlocutory injunction against Sandoz, restraining it from selling biosimilar rituximab (Riximyo) in Australia until 11 August 2019. The 4...
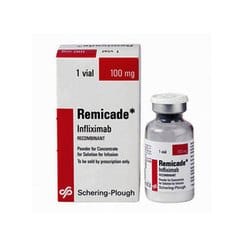
J&J suffered another blow today as a key Remicade® (infliximab) patent was revoked by US Court of Appeals (Federal Circuit). Upholding the USPTO decision on reexamination...
Biosimilar development is not for the faint hearted. The time and costs involved in R&D, clinical, and regulatory are exorbitant, and the patent barriers to launch are...

IP Australia is inviting submissions by 17 November 2017 on the first wave of IP reform initiatives arising from the Government's response to the Productivity...

Eleven years after the launch of the first biosimilar product in EU, we are now in a position to calibrate our biosimilar market expectations with market experience, rather than...
In a case relating to patents for global blockbuster biologic Humira™ (Adalimumab), the Full Federal Court has drawn a bright line around the limitations of the PTE regime in...

This afternoon, the Australian Government released its response to the report of the Productivity Commission Inquiry into IP released 20 December 2016. In its 25 page response to...
Subscribe to our Pearce IP Blogs and BioBlast® to receive our updates via email.