Pearce IP recently reported on the Full Court of the Federal Court decision in Ariosa Diagnostics, Inc v Sequenom, Inc [2021] FCAFC 101, which confirmed that a method of detecting cell-free foetal DNA (cffDNA) in pregnant women, permitting non-invasive prenatal diagnosis, is patent eligible in Australia. However, the same invention was found to be patent ineligible under US law. This is not the first time that Australian and US courts have differed in relation to the patent eligibility of life sciences inventions, particularly gene-based inventions.
The following table details the patent eligibility of gene-based inventions and inventions that rely on a naturally-occurring phenomenon under the current US, Australian and European laws.
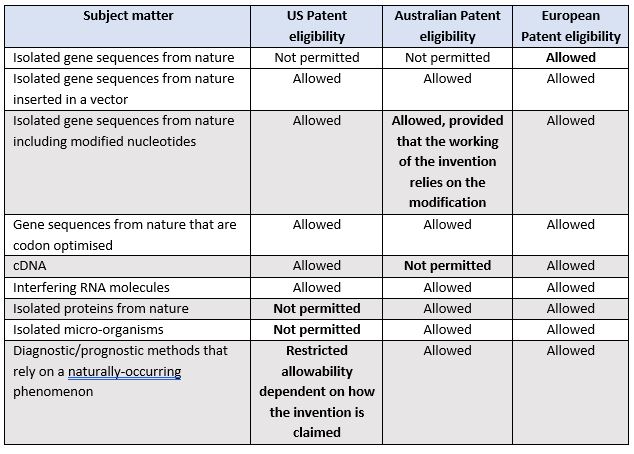
Given the differences in what is considered patent eligible subject matter in the US, Australia and Europe, life sciences inventors and their patent attorneys need to understand the relevant patent laws to ensure that the most commercially relevant scope of protection for life sciences inventions is ultimately obtained in these jurisdictions.

